Anxiety disorders are common, debilitating psychiatric syndromes and include panic disorder (PD), post-traumatic stress disorder (PTSD), and generalized anxiety disorder (GAD). The investigators of the FNL have studied these diseases, including as part of a multi-site Center for Neural Systems of Fear and Anxiety (Dr. J. LeDoux, PI). Translational models have been used to integrate human work with animal models of fear and anxiety, and complementary neuropsychological paradigms which address both top-down and bottom-up mechanisms have been utilized, with a focus on abnormalities of front-limbic-striatal function.
Findings in PTSD and Panic Disorder
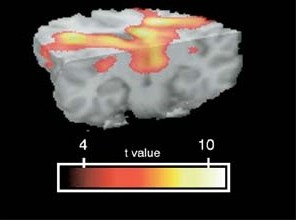
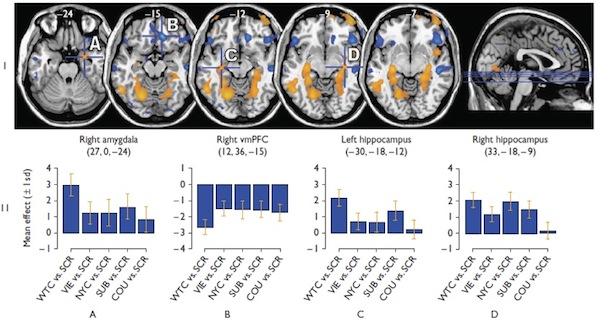
The FNL has examined both function and structure in anxiety disorder patients. For example, studies in PTSD show that these patients, compared with normal control subjects, demonstrate abnormal amygdalar activation during the processing of PTSD-related linguistic stimuli specifically, and that this occurs in a time-dependant fashion. In panic disorder, there is an abnormal size of the brainstem, an important finding given the neurotransmitter-rich projections arising from this structure which are integral to the bodily responses associated with panic attacks. Direct comparison of brain activity patterns in panic disorder compared with PTSD has shown that compared to PTSD patients and healthy controls, panic disorder patients showed significantly less activation to threat stimuli and increased activation to safe stimuli in the subgenual cingulate, ventral striatum, and extended amygdala, as well as in the midbrain periaqueductal grey region, suggesting potentially unique neurocircuitry subserving distinct anxiety disorders.
Cortisol and Brain Activity
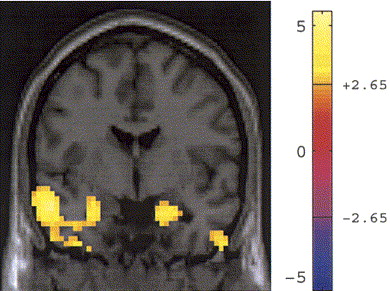
Many psychiatric and neurological disorders have been associated with dysregulation of the HPA axis. Frontolimbic structures involved in fear conditioning have also been associated with HPA-axis modulation, but little work has examined the direct associations between these circuits and neurohormonal functions using fMRI. The relationship between typical diurnal cortisol reactivity and fronto-limbic brain activity in response to stressful stimuli helps to link our understanding, in humans, of the relationshiop between the activity of stress hormones and brain activity in regions that modulate, and are modulated by, that stress response.
Sex Differences in Fear/Anxiety
Given the difference in the prevalence of anxiety disorders between the sexes, it is important to examine differential underlying neurobiological mechanisms, as this may lead to sex-specific interventions. Sex differences in anticipatory anxiety in healthy subjects have been identified, using an instructed fear paradigm, with females showing greater activity in the subgenual anterior cingulate cortex, and the functionally-related regions of the insula and brainstem . This finding suggests a neural substrate for the greater susceptibility of women to anxiety disorders.
Fear and Anxiety in Healthy Subjects
Our studies have helped to further elucidate the neurobiological changes underlying the normal human fear/anxiety response (5). Using an instructed fear conditioning paradigm, we have identified a pattern of decreased activity in primary motor cortex with increased activity in dorsal basal ganglia to the anticipation of aversive electrodermal stimulation, possibly representing a state of motor readiness in response to danger. The basal ganglia are a critical emotion/motor interface that allow the translation of emotional information into behavioral responses. Amygdalar activity is only present in the earliest period of threat, which is consistent with previous literature on instructed fear, where habituation of this response is described.
1. Protopopescu X, Tuescher O, Pan H, Goldstein M, Cloitre M, Gorman J, Ledoux JE, Engelien W, Silbersweig D, Stern E: Differential time-courses and specificity of amygdala activation in PTSD subjects and normal controls. Biological Psychiatry, 57: 464-473, 2005.
2. Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien A, Yang Y, Gorman J, LeDoux J, Stern E, Silbersweig D. Increased brainstem volume in Panic Disorder: a voxel-based morphometric study, Neuroreport 17: 361-363, 2006.
3. Tuescher O, Protopopescu X, Pan H, Cloitre M, Butler T, Goldstein M, Root JC, Engelien A, Furman D, Silverman M, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential activity of rostral cingulate and brainstem in panic disorder and PTSD. J Anxiety Disorders. 2010 Oct 10; 1201: 1-7. In press.
4. Butler T, Pan H, Epstein J, Protopopescu X, Tuescher O, Goldstein M, Cloitre M, Yang Y, Phelps E, Gorman J, Ledoux J, Stern E, Silbersweig D. Fear-related activity in subgenual anterior cingulate differs between men and women. Neuroreport 16: 1233-1236, 2005.
5. Butler T, Pan H, Tuescher O, Engelien A, Goldstein M, Epstein J, Weisholtz D, Root JC, Protopopescu X, Cunningham-Bussel AC, Chang L, Xie X, Qiang C, Phelps E, LeDoux J, Stern E, Silbersweig D. Human fear-related motor neurocircuitry. Neuroscience, 150: 1-7, 2007.
6. Root J, Tuescher O, Pan H, Epstein J, Altemus M, Cloitre M, Silverman M, Furman D, LeDoux J, McEwen B, Stern E, Silbersweig D. Association between fronto-limbic function and cortisol levels in response to stressful stimuli, Neuroreport. 2009 Mar 4;20(4):429-34
7. Cunningham-Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, Weisholtz DS, Pavony M, Silverman ME, Goldstein MS, Altemus M, Cloitre M, LeDoux J, McEwen B, Stern E, Silbersweig D. Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology. 2009 Jun;34(5):694-704. Epub 2009 Jan 9.